Is Bromine a Good Conductor of Electricity
Generally non-metals are bad conductors of electricity but graphite is an exception. Ionic compounds cannot conduct electricity when solid as their ions are held in fixed positions and cannot move.
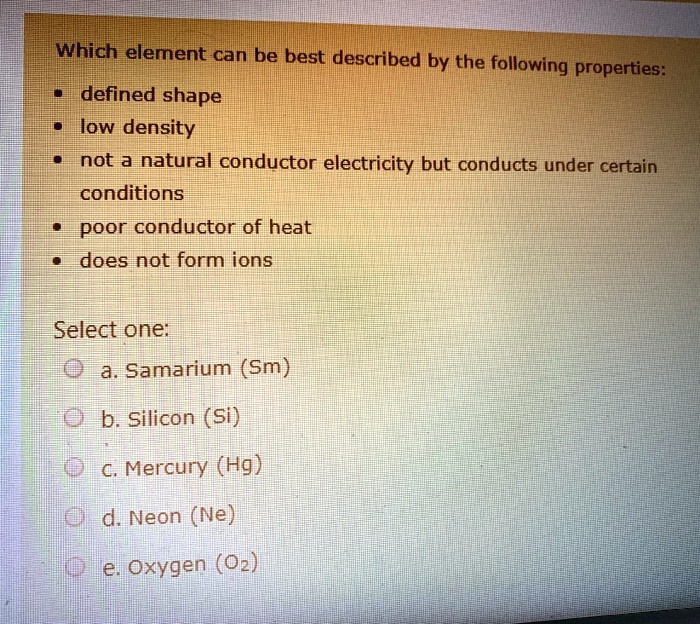
Solved Which Element Can Be Best Described By The Following Properties Defined Shape Hlow Density Inot A Natural Conductor Electricity But Conducts Under Certain Conditions Poor Conductor Of Heat Does Not Form Ions
Mercury has the highest Thermal Conductivity in comparison to Iodine Bromine and Alcohol.
. Silicon is a semiconductor meaning that it does conduct electricity. A Li b K c Rb d H e all of these are correct. Bromine is a very poor conductor of electricity.
An insulator is a material which does not allow the current to pass through it. Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard. C Graphite is the allotropic form of carbon and it is a good conductor of electricity.
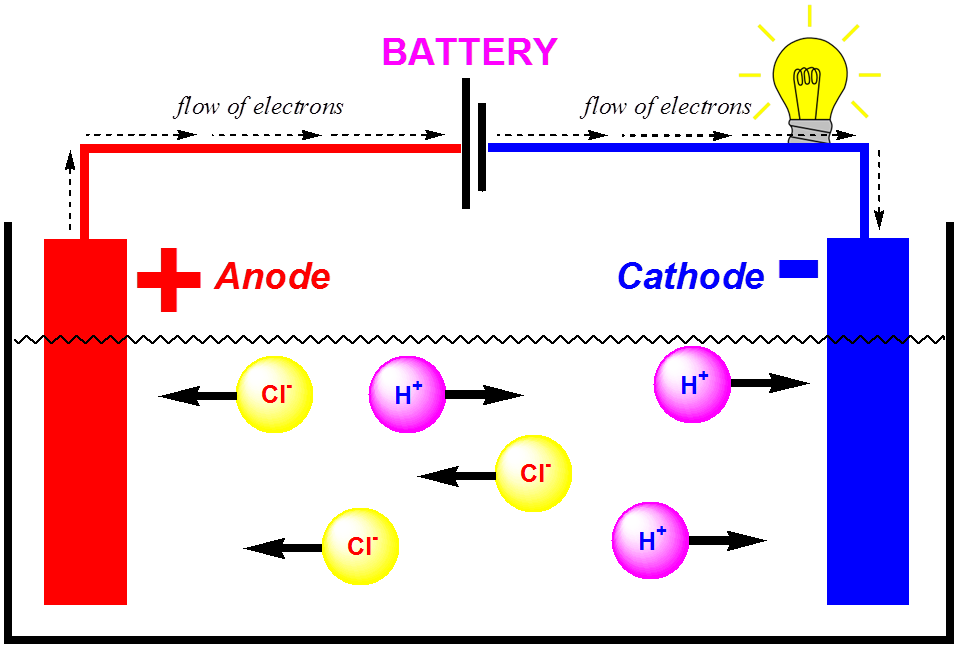
Yes bromine is the only liquid non-metal known at room temperaturedue to which ions of Br are produced which can conduct electricity along with Na ions. Is magnesium a good conductor of electricity. Which of following is not good conductor of electricity.
V Kerosene does not decolourise bromine water while cooking oils do. Chlorine is a non-metal substance and hence it is a bad conductor of heat and electricity. This happens mainly due to two reasons.
Is chlorine a good conductor of heat and electricity. A conductor is a material which allows the current to pass through it. Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard.
CAluminium is a good conductor of heat. Is silicon a conductor. Bromine Thermal Conductivity.
Diamond is also made up of carbon but it is a bad conductor of electricity. The chemical reactivity of an element is determined by which of the following. Graphite is a non-metal.
But still Br is a non-metal thus its conduct electricity to very small extent. Bromine is not a good conductor of electricity. Bromine as the element is called on its own is a nonmetal and is a poor conductor of electricity.
Generally non-metals are bad conductors of electricity but graphite is an exception. Which liquid metal is the best conductor. Graphite Phosphorus Hydrogen Bromine 1 See answer AadhiraiA is waiting for your help.
The other options- iodine sulphur and bromine are non-metals. The Correct Answer is Bromine. Is bromine a conductor.
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity k or λ measured in WmK. The atomic number of Bromine is 35. Add your answer and earn points.
Thermal conductivity of Bromine is 0122 WmK. It is a p-block element. Bromine is a very poor conductor of electricity.
Bromide salts conduct electricity if dissolved in. It is a measure of a substances ability to transfer heat through a material by conduction. Due to this property it is used in making of pencil cells.
Iii Graphite is a good conductor of electricity. BCopper is a good conductor of electricity. Examples of conductors- copper silver graphite etc.
Bromine is a very poor conductor of electricity. Which element is most likely to be a good conductor of electricity. It is a deep red noxious liquid at room temperature.
Which element is not an alkali metal. Fluorine is a bad conductor of electricity as it remains incapable of giving out free electrons that remain localized on fluorine. Which is good conductor of heat mercury or alcohol.
Bromine is a non-metal which exists as a liquid at room temperature. Examples of insulators- bromine paper plastic wood etc. DBromine is the only liquid non-metal.
Because the structure of graphite is in such a way that electrons can move freely and conduct electricity. Iv Acetylene bums with a sooty flame. Graphite is a non-metal.
Unlike a typical metal however silicon gets better at conducting. Hence the correct answer is option B. The reactivity of fluorine atom is high as it needs only one electron to fill its outermost orbit.
Hence they are bad conductors of electricity. Can bromine conduct electricity. By November 3 2020 Comments Off on is bromine a good conductor of electricity Which element is a metal that is in the liquid phase at STP Which element is malleable and a good conductor of electricity at STP Which element is a brittle solid with a low conductivity at STP Which element exists as a diatomic molecule at STP.
Since the ions or electrons in a non-metal are bonded with covalent bonds therefore their electron does not. Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard. Due to this property it is used in making of pencil cells.
This structure means that bromine is a very poor conductor of electricity with a conductivity of around 5 10 13 Ω 1 cm 1 just below the melting point although this is higher than the essentially undetectable conductivity of chlorine. Which of the following non metal is a good conductor of electricity. The Correct Answer is Bromine.
That runs below boron B silicon. Which element is chemically similar to lithium. Because the structure of graphite is in such a way that electrons can move freely and conduct electricity.
Bromine at regular temperature is a gas which will not conduct electricity but when bonded with other elements it will conduct electricity easily. Not all the substances of carbon are good conductors of electricity. The Correct Answer is Bromine.
It is the third-largest halogen. Therefore graphite is a good conductor of electricity. Which is not a good conductor of electric.
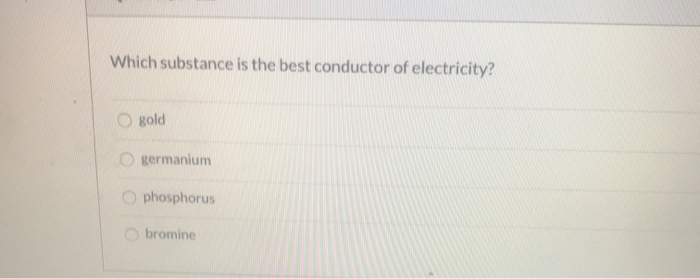
Solved Which Substance Is The Best Conductor Of Electricity Chegg Com

Properties Siyavula Textbooks Grade 10 Physical Science Caps Openstax Cnx

Electrical Conductivity Across Period 3 Creative Chemistry

Gcse 1 9 Why Can Ionic Compounds Only Conduct As A Liquid Youtube

Relationship Electric Current Potential Difference 1 Relationship Current Different
Conductivity The Periodic Table

Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T

Selina Concise Chemistry Class 7 Icse Solutions Chapter 6 Metals And Non Metals Learn Cram Https Www Teaching Chemistry Chemistry Lessons Chemistry Basics

Categories Of Elements Metals Good Conductors Of Heat And
What Substances Are Used To Conduct Electricity Quora

Can Bromine Conduct Electricity
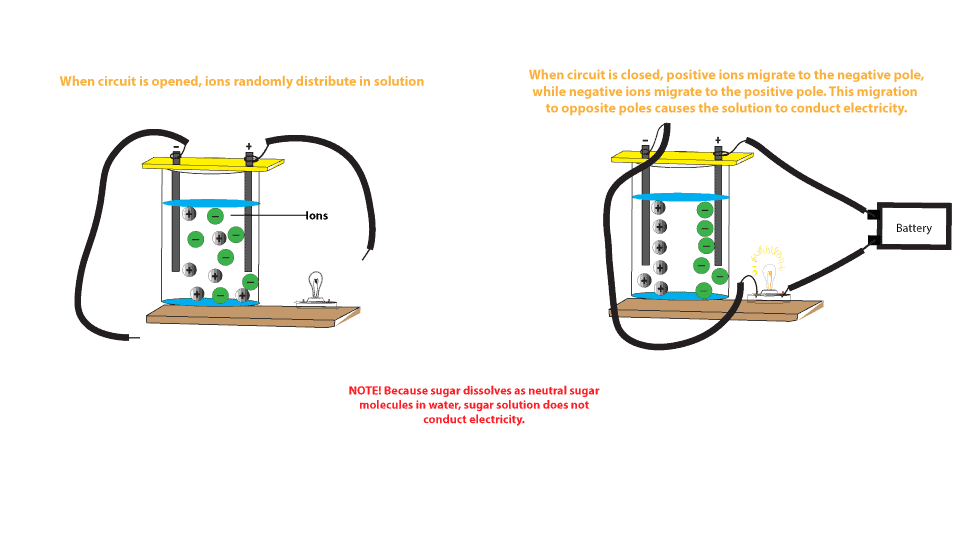
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T

Solved Which Element Is Most Likely To Be A Good Conductor Chegg Com
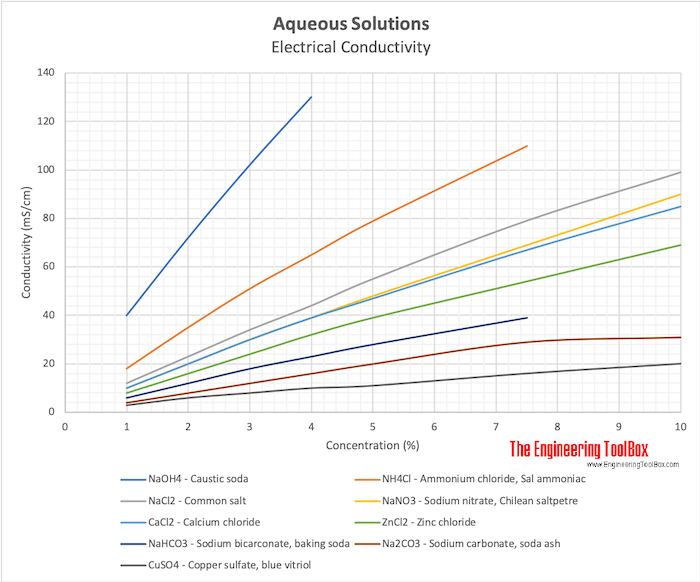
Electrical Conductivity Elements And Other Materials
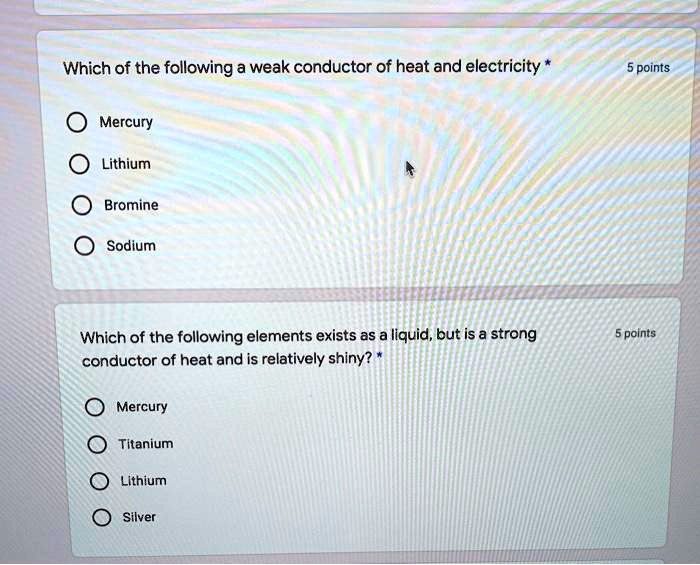
Solved Which Of The Following A Weak Conductor Of Heat And Electricity 5 Points Mercury Lithium Bromine Sodium Which Of The Following Elements Exists As Liquid But Is A Strong Conductor Of Heat

Comments
Post a Comment